The CMS Fall Conference Highlights
The Centers for Medicare & Medicaid Services (CMS) annual fall conference never misses in providing important information for Sponsors. This year’s conference was held in Baltimore on September 6, 2018. Conference materials can be found on the Compliance Training, Education & Outreach website here. CMS covered many areas, but here I’m focusing on two key areas of the conference: Medicare Communications and Marketing Guidelines (MCMG) updates and appeals and grievances classification best practices.
MCMG
One of the most important clarifying statements from CMS was definitively communicating the MCMG replaces the Medicare Marketing Guidelines (MMG) and any guidance within it. What does that mean? If previous MMG guidance is not dictated in the MCMG, Sponsors should consider that guidance obsolete. While many had assumed that was the case, it’s always better to get clarity straight from the source. This single statement should resolve many looming questions by Sponsors.
Of equal significance was CMS further outlining the meaning of “marketing” material. In order to be considered “marketing,” the material must meet both intent and content requirements. If only one of these distinctions is met, the material would not be considered “marketing.” The example used to help drive this message was the Evidence of Coverage (EOC). The EOC has content that would meet “marketing” definitions, however, the intent is not to persuade a beneficiary to enroll, so it is considered a communication. This new guidance will eliminate much burden for Sponsors in relation to the number of materials requiring CMS review and submission. While this removes some administrative burden, the oversight may need to be more stringent as health plans are now even more responsible for correct classification of documents and ensuring documents still comply with all CMS requirements. Additionally, documents must be available upon request.
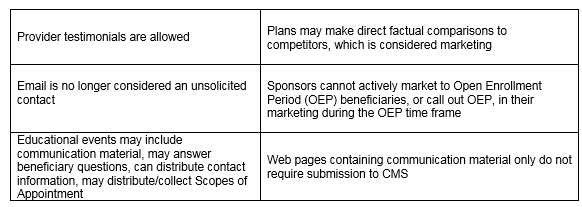
Other noteworthy clarifications from CMS:
An updated MCMG was distributed by CMS on September 6, 2018.
APPEALS & GRIEVANCES
In their continued effort to help Sponsors in the appeal and grievance space, CMS and Sponsor representatives presented classification best practices. CMS reminded Sponsors of key words when classifying appeals and grievances:
Inquiry ----- a question
Grievances ----- a dissatisfaction
Coverage/Organization Determination ----- a decision to provide or pay
Appeal ----- a dispute of a previous decision
Several health plan speakers shared their best practices. Some of the valuable recommendations that changed their organization included:
- Centralized Customer Service staff focused on identifying appeals and grievances. Staff training focused on real-life examples and involved frequent refreshers.
- Cross-functional all staff on grievances, coverage and organization determinations, and appeals. Training not only included correct identification and classification but also the downstream member and CMS impact of failure to correctly identify complaints. This holistic approach supports the member experience and greater CMS compliance.
- Intake staff who review cases quickly to identify any potential misclassification of cases. Root cause analysis as a routine part of processing. So often the focus is on resolving the identified issue for that member, which is critical. Equal importance is remedying any systemic concerns to prevent the problem from impacting other members. The presenters discussed how root cause was discussed in routine meetings with other departments for brainstorming and resolution.
- Embedded Compliance staff within the business units. A great trend in the industry in recent years is to have an operational Compliance person located in critical operations units. They are available to help staff work through complex issues where a keen eye on regulatory guidance is needed. This keeps cases moving forward and allows staff to feel supported all while maintaining high compliance.
CMS conferences have always been valuable resources in clarifying new changes or functions that have the attention of CMS. Don’t miss utilizing this valuable resource to stay current.
 Gorman Health Group provides training tools, conducts mock audits, and supports process improvement initiatives related to Parts C & D appeals and grievances? We also provide interim staff for clients when they have a need in this area.
Gorman Health Group provides training tools, conducts mock audits, and supports process improvement initiatives related to Parts C & D appeals and grievances? We also provide interim staff for clients when they have a need in this area.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Barriers to the New Opioid Care Coordination Safety Edit for 2019
The Contract Year (CY) 2019 Call Letter and Centers for Medicare & Medicaid Services (CMS) expectations for all sponsors to implement a real-time opioid care coordination safety edit at the time of dispensing as a proactive step to engage both patients and prescribers about overdose risk and prevention will require:
- Implementing a point of sale (POS) safety edit days’ supply limit of 7 days for initial fills of opioids (for opioid naïve patients).
- Implementing an opioid care coordination soft POS safety edit at 90 Morphine Milligram Equivalent (MME) per day.
- In implementing this edit, sponsors should instruct the pharmacist to consult with the prescriber, document the discussion, and if the prescriber confirms intent, use an override code that specifically states the prescriber has been consulted.
- Sponsors will have the flexibility to include a prescriber and/or pharmacy count in the opioid care coordination edit.
- Sponsors will also have the flexibility to implement hard safety edits and set the threshold at 200 MME or more and may include prescriber/pharmacy counts.
These new requirements have created an urgency for the National Council for Prescription Drug Programs (NCPDP) to evaluate guidance that could possibly require new reject codes and override codes for POS safety edits. The NCPDP Telecommunication Standard is the standard used for eligibility, claims processing, reporting, and other functions in the pharmacy services industry as named in the Health Insurance Portability and Accountability Act (HIPAA) and in electronic prescribing as named in the Medicare Modernization Act (MMA). With the expectation the opioid care coordination edit should be implemented in 2019, there is very little time for the industry to develop and implement a standardized communication process for these safety edits where there are variations in the exception criteria.
The opioid care coordination edit will allow pharmacists to play a greater role in patient care, but this will require the development of standardized electronic edits and documentation to facilitate POS rejects that currently require a coverage determination initiated by the prescriber. In addition, the intent of the POS soft safety edits to bypass the coverage determination process for clinical safety edits could create audit risks for the pharmacy without the additional POS codes to override soft edits in opioid care coordination. An added burden, given the implementation time frame, is the CMS expectation that sponsors’ network pharmacies and customer service representatives be adequately trained with regard to these new care coordination edits. NCPDP hopes to work with CMS in identifying effective, less burdensome approaches using industry adopted standards that will yield safer and appropriate opioid prescribing and ensure patient access to care and alleviate any confusion on the intent of the soft and hard POS edits proposed in the CY 2019 Call Letter.
On one hand, the national opioid crisis is urgent, and relying on technology to contain the crisis is essential, however, the NCPDP Telecommunication and SCRIPT standards do not currently support the edits and the necessary documentation that needs to be captured between the prescriber and the pharmacist. There will be a need to standardize this across the industry for opioid MME claim rejection and override codes. In the meantime, stakeholders would need to rely on manual attestation and documentation processes. Plan sponsors will have to review their current opioid policy to ensure a variety of tools are in place to address all the issues and to ensure their pharmacy benefit managers (PBMs) and call centers are ready in 2019.
Gorman Health Group can help in developing effective, proactive utilization management programs for Part D opioid utilization. Our experts are available to assist in the design of a comprehensive opioid overutilization prevention program that can address member, provider, and pharmacy care coordination policy per CMS guidance and expectations.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
How to Avoid Network Development & Expansion Pitfalls
Network management, directory accuracy, service area expansions (SAEs), and the new triennial review process have placed many Medicare Advantage (MA) plans in flux and exposed serious issues – from policy to process to staffing and technology. As we move into the countdown for network development and expansion for 2020, a review of lessons learned will be helpful in planning and avoiding some of the pitfalls from 2019.
- In previous years, plans submitted their Health Service Delivery (HSD) tables with their applications, and, by the end of April, there was clear insight into which counties the Centers for Medicare & Medicaid Services (CMS) deemed to have an adequate network. This year provided a new challenge with bids being submitted prior to HSD tables being uploaded and reviewed by CMS.
As multiple new regulations on network adequacy and directory accuracy have been implemented, a lesson learned by many is there can never be too much communication and oversight in managing provider network contracting and credentialing data.
- Overall project management and poor communication between outside vendors during an expansion can lead to stressful situations and put the plan at risk for compliance actions. Planning ahead and including oversight and transition plans at the start of any project can lessen the risks down the road.
Here at Gorman Health Group, we have been brought in and have identified situations described and have outlined extensive work plans to include policy and procedure (P&P) changes, technology updates, network development plans, compliance solutions, and assisted plans in working through the corrective action needed.
We can’t stress enough how the cost of prevention and planning on the front end becomes even more cost effective when faced with correcting on the back end.
Summer is the best time to be proactive and start planning your next SAE or even your first step into the MA world. Alternatively, perhaps, summer offers just the right amount of downtime to start planning for updated oversight policies.
As you start your initial or expansion planning process and set new network monitoring processes in place to ensure preparedness, consider this: Gorman Health Group has a long history of providing the following:
- Leveraging long-standing relationships and nationwide experience coupled with a cost-effective team of Senior Consultants and Network Analysts to effectively stand up a contracted provider network
- Designing and developing a network strategy and product strategy that take into account the quality, financial, risk adjustment, and Star Ratings goals for success within the competitive landscape of your market(s)
- Developing the oversight and monitoring P&Ps needed to address the new network and directory requirements
- Preparing plans’ HSD tables for a CMS filing or bid submission as well as preparing network exceptions to include all the required elements.
Let us know how we can work together now to support your plan’s goals for the future. We’ve been there, fixed that, and will ensure your success. Contact me directly at emartin@ghgadvisors.com.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Webinar Recap: 2019 MCMG Highlights
Thank you to all who came to the GHG webinar regarding the 2019 Centers for Medicare & Medicaid Services (CMS) Medicare Communications and Marketing Guidelines (MCMG). We had a great attendance and are working to answer all of your questions and get a Q&A to all who attended plus the presentation! We wanted to take a minute to discuss a few items where GHG received a large number of questions: mailing statement disclaimers, Open Enrollment Period (OEP) beneficiary plan changes, and website review.
Mailing Statements
There are now only two mailing statements in Appendix 2 of the Disclaimers. The first is for Plan Information, and this is causing the most confusion. The disclaimer states the required text is “Important [Insert Plan Name] Information”. GHG interprets this to mean this disclaimer only needs to be on your member’s important plan information such as enrollment, benefit, operations, and other important plan information. GHG does not believe CMS intends advertising mailings to use this disclaimer as it would be confusing and misleading to prospects.
In addition, GHG contacted CMS about the requirement to insert the plan name since there was confusion about whether the plan needed to utilize the plan benefit package (PBP) plan name vs. the Medicare Advantage Organization (MAO) name and whether this was even necessary since the name of the plan is already on the envelope. GHG’s interpretation of CMS’ response is that as long as the name of the MAO is on the envelope and is recognizable to the member as their health plan, then inserting the plan name is not needed.
OEP
There was some confusion regarding the plan types a beneficiary can select during the OEP. A beneficiary is allowed a one-time change. The following chart describes the allowed changes:
- Medicare Advantage Prescription Drug plan (MA-PD) to MA-PD
- MA-PD to MA-only plan
- MA-only to MA-PD plan
- MA-only plan to MA-only plan
- MA-PD to Original Medicare (with or without Part D)
- MA-only plan to Original Medicare (with or without Part D)
Website = New Guidance From CMS That Will Make You Smile!
Those in Original Medicare or Original Medicare with Part D are not able to enroll in an MA-only or MA-PD plan. In addition, the OEP is not available for those enrolled in Medicare Savings Accounts, Cost plans, or Program of All-Inclusive Care for the Elderly (PACE) plans.
On Friday, May 10, CMS released an email blast, “Updates to the Website Requirements in the Medicare Communications and Marketing Guidelines.” CMS is no longer requiring website marketing content to be submitted into the Health Plan Management System (HPMS) for a 45-day review. Plans can now submit their websites via HPMS as a Word document that contains a URL (sound familiar?). Screenshots, test sites, etc., are not needed. Plans are permitted to submit the website as File & Use – both their own and/or third party websites. This means all websites must be submitted five days before going live, so mark your calendar! In addition, CMS added guidance about the Material ID and websites. All website pages must have a Marketing ID, but the ID does not need to include “M” or “C.”
When making website updates, the plan must include the website’s URL in a Word document and include a summary of the changes. The updates may go live five days after HPMS submission. Plans do not need to take down their website while making updates, but make sure the changes do not go live until five days after the HPMS submission.
CMS has stated another version of the 2019 MCMG will be released before the end of the summer. I am hoping for more clarifications to occur. Let us know how we can help you!
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
CMS Takes Action to Lower Drug Prices, Permits Step Therapy
I have been known to utter “holy cow” in my day, and the recent Centers for Medicare & Medicaid Services (CMS) Health Plan Management System (HPMS) memo allowing step therapy for Part B drugs was the reason for my most recent exclamation. Who saw this coming?
The first sentence of the memo reads, “CMS is hereby rescinding our September 17, 2012, HPMS memo, “Prohibition on Imposing Mandatory Step Therapy for Access to Part B Drugs and Services.” Holy cow. For some time, the Part B versus Part D issue has been the bane of Medicare Advantage (MA) plans. CMS has tried over the years to ease that pain by issuing guidance on when a drug can be billed as Part B and when it should be billed as Part D. As late as the 2017 Call Letter, CMS reminded us of the new 2016 requirements at 42 CFR §422.112(b)(7), which require all plans to establish and maintain “a process to ensure timely and accurate POS transactions, and to issue a decision and authorize or provide the benefit as appropriate under Part B or Part D when a party requests a coverage determination.” So why the about-face on requiring expediency in dispensing Part B drugs? It all comes down to cost containment.
Step therapy has been a mainstay in Part D drug utilization management to help contain the cost of medications. The idea is for the beneficiary to use an effective and less expensive drug before moving onto more costly therapies. This makes good sense. Why pull out a flame-thrower when a match will work? Historically, Part B drugs have not been subject to step therapy – providers either pulled their preferred drug from the shelf or wrote a prescription the pharmacy filled under Part B. Now Part B drugs will be subject to the same coverage determinations and appeals processes for step therapies Part D drugs have enjoyed. How might this affect what providers stock in their offices? And how about Home I.V. and Long-Term Care pharmacies? These pharmacies have had the luxury of dictating what products would be used in the past, and Part B step therapy will be a game changer as plan sponsors take back control over what is dispensed. Holy cow.
While cost containment is desired, there are also unintended consequences. Beneficiaries may experience a delay in obtaining their drugs due to determinations and appeals processes. Part D drugs are subject to coverage determinations that have a 72-hour time frame for standard determinations and 24 hours for expedited determinations. Part B drugs are subject to organization determinations. These Part B pre-service determinations have a 14-day time frame for standard requests and a 72-hour time frame for expedited requests. In last night’s memo, CMS “strongly encourages that MA plans expedite requests for exceptions in Part B to align with the 72-hour adjudication timeframe for requests in Part D.” Holy cow. Plan sponsors will need to implement a procedure for their organization determinations, appeals, and grievances staff so drugs can be given priority over other requests.
Plan sponsors will also have new Part B issues to deal with. The memo calls out the need for “interactive medication review to discuss all current medications,” which may be code for Medication Therapy Management (MTM). How will these new requirements affect MTM programs and targeting of eligible beneficiaries? Chapter 7, Section 30.2 (2) and (3), of the Medicare Prescription Drug Benefit Manual speaks to targeting Part D drugs only for both numbers of drugs being taken and dollar amount thresholds. Is the expectation that Part B drugs be added to these targeting thresholds?
And last but not least is the job of actually developing Part B step therapy criteria. Local Coverage Determinations (LCDs) and National Coverage Determinations (NCDs) will still apply, and we know beneficiaries already using a Part B drug will be grandfathered in. CMS is allowing “cross-contamination” of steps. Plan sponsors may require a beneficiary to use a Part D drug before getting a Part B drug and vice versa, requiring a Part B drug before receiving a Part D drug. Can step therapies incorporate both Part B and Part D drugs within a specific step? And who will review exception requests – the organization determination team or the coverage determination team – when both Part B and Part D drugs are incorporated into step therapies? Thinking about the possibilities is absolutely mind-numbing.
And, of course, the window for uploading these new step therapies is fast upon us – August 17 through August 21. Holy cow. So get those Pharmacy & Therapeutics Committees cracking! And while you’re at it, you may want to check with your Marketing Department to see how far along you are in the development of your Annual Notice of Change and Evidence of Coverage. If you can’t hold the presses to add these new step therapies, you will need extra time to develop addendums to be sent to your beneficiaries.
I am looking forward to seeing how this new twist to an already complex benefit will play out in 2019. As always, if you need help navigating the rules, regulations, and best practices, Gorman Health Group has the experts to help you.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Top 10 Changes – NEW Medicare Communications and Marketing Guidelines (MCMG)
Gorman Health Group will conduct a webinar on the new guidelines in the next couple of weeks, but we wanted to get this out right away as the Centers for Medicare & Medicaid Services (CMS) has rewritten the Medicare Marketing Guidelines (MMG) for 2019. A lot of information is the same but just condensed – there are a lot changes! Here is the top 10 list of 2019 CMS MCMG changes:
- CMS has grouped activities and materials into Communications and Marketing that are distinguished based on intent and content. It will be important to understand the differences and educate your staff since interactions with beneficiaries can start out as a communication and end up as a marketing communication – with different compliance requirements.
- Plans/Sponsors may compare their plan to another plan/sponsor provided they can support the comparison through studies or statistical data and the comparisons are factually based. CMS does not provide any detail on the scope of studies or time periods required for statistical data, so proceed with caution if considering publishing plan comparisons.
- Plans cannot market during the Open Enrollment Period (OEP) or engage or promote agent/broker activities to target the OEP as an additional marketing opportunity but can do the following:
- Market to beneficiaries who are new to Medicare, also known as “age-ins,” who have not yet made an enrollment decision
- 5-star plans can continue the Special Enrollment Period (SEP)
- Market to dual-eligible and low-income subsidy (LIS) beneficiaries
- Send marketing materials and have meetings with those who request the information/meeting, and provide OEP information via the call center
- CMS has provided detailed guidance on activities in the healthcare setting with an emphasis on differentiating between activities a provider (or pharmacist!) performs as a matter of a course of treatment versus activities a plan or provider performs aimed at influencing an enrollment decision. If you recall, this was one of the specific topics for consideration for which the agency sought feedback in its April 12, 2018, request for input on this guidance.
- There is a new Material ID process – plans must use a “C” for communication materials or “M” for marketing materials at the end of the Material ID. Here is an example: H1234_abc567_C.
- Documents such as the Summary of Benefits (SB), Evidence of Coverage (EOC), Annual Notice of Changes (ANOC), directories, and formularies need to be posted by October 15. CMS has listed each required document in a chart that describes to whom required, timing, method of delivery, Health Plan Management System (HPMS) timing, format, additional guidance, and translation requirements. This is very helpful.
- Plans no longer have to mail the EOC to existing enrollees, but the ANOC must be mailed. If a new member enrolling throughout the year (for example, for a June 1 effective date) requests hard copy materials to be mailed to him/her, the hard copy request must be fulfilled within three business days, and the request remains in effect until the member leaves the plan or requests that hard copies be stopped.
- Plans must keep their call centers open 7 days a week, from 8 a.m. to 8 p.m., for an additional 6 weeks – from February 14 to March 31. These additional costs need to be factored into next year’s budget, and sponsors should be having conversations with any call center vendors supporting this line of business.
- The rules for disclaimers have changed again, but now it’s easier! Make sure you read each disclaimer since parts of some disclaimers are still needed or if you mention 10 or more benefits.
- Plans must submit website marketing content for review, including contracted third-party websites. Plans do not need to submit web pages with or containing CMS-required content for review. This is a 45-day review! This means almost every website needs to be reviewed now. Website content that has not been reviewed/approved cannot be viewable to the public. This is a significant change as sponsors were previously permitted to post websites that were pending review.
This is not a year to skim through the guidelines—we are still reading and re-reading the document to make sure we understand the implications. Good luck, and watch for our webinar in the upcoming weeks!
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Focus on Rural Population: What Your Plan Still Has Time to Do
Here we are at the end of July already! Time flies, especially when we are busy preparing for enacting our bid submission approvals and planning for rollout of plan year 2019 activities and new members. It is not too late to still enhance this year’s activities and positively affect our members within the remaining five months of this plan year, especially in the rural areas of your plan’s service area. CMS released its first "rural health strategy" here: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2018-Press-releases-items/2018-05-08.html
Barriers to care/access and disparities for the rural service areas and their communities are routinely missed as a focus for a care management program objective, a quality improvement process, or independent study within population health management.
Here are a couple of tips to consider and questions to ask yourself as a plan that can truly be implemented within this plan year:
What do you know (or not know) about your rural populations specifically?
Age bands overlaid by claims data/GeoAccess: Oftentimes, populations in rural areas are older than those residing in urban areas. This means access or capability to access care is a potential issue right off the bat. Elderly populations who may be isolated by a rural geographic location due to distance to care can be compounded by other issues: daylight hours available to drive, their own vision, condition of their vehicle, if they have to care for others…you get the picture. Do we as an industry really take into account how to identify those who are isolated by being rural? I believe we can do better!
Plans could take their specific rural counties and break down by age bands the populations who live there; overlay the claims utilization to determine patterns of care AND potential barriers. For example, if you have vision as a supplemental benefit, and you know your elderly population in the rural service area cannot access the vision stores due to the fact they are all urban, how do you expect these members to access care SAFELY simply by having the vision benefit? What can you consider to support these folks? This is where telemedicine could become your new best friend to support the reach your network cannot. I believe plans could use the telemedicine option more than we see today. Many plans are not aware of the details, the codes, and what the benefits are, so please educate your network teams, provider networks, and update your care management program to include this option. If you are not sure what the rules are, look here: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf.
Also, consider engaging a home visit vendor to support this population – you will want to make certain that networks can deliver in the rural areas and not face access to members’ issues.
If your plan does have rural hospitals that service your rural counties, please be certain to mine this facility’s utilization, emergency room, observation, and inpatient data. Frequently, rural hospitals serve communities with greater rates of diabetes and known associated hypertension and obesity, all of which speak to the rural community structure and lack of urban services.
Don’t forget the analysis of rural service area prescription drug claims. Drug claims alone often identify issues for and about plan members that may not otherwise be exposed.
Introduce “rural service area access” into your quality program as a quality improvement project. Because rural communities face provider shortages, especially primary care, as well as behavioral health, dental, and vision, consider enacting a rural clinical day, either through a Federally Qualified Health Center (FQHC) or other partner to draw members to a one-stop shop day of service. Sort of like a spa day but for health! If folks cannot get there, offer transportation, too!
Thinking outside the box to enhance our rural populations’ access, engagement, and health outcomes could only benefit everyone. If you need assistance to evaluate your plan’s populations, creative care model changes, please reach out to me at jscott@ghgadvisors.com.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Trump Administration Alarms PBMS with First Drug Pricing Initiative
The Department of Health and Human Services (HHS) issued its blueprint for dealing with the high price of prescription drugs in May, and the administration is finally undertaking a number of initiatives to operationalize the proposals. So far, as seen from the updates below, the proposals will have the biggest impact on pharmacy benefit managers (PBMs) and payers.
Voluntary Price Drops
Several weeks ago, President Trump tweeted that Pfizer was not following the intent of the blueprint and actually met with the Pfizer CEO at the White House to put pressure on the drug company to delay price increases. As a result, Pfizer will delay price increases on 40 drugs from July until early in 2019. Last month, Novartis postponed a price increase for its autoimmune drug Cosentyx in response to California’s new drug transparency pricing program. The Novartis CEO also announced this week the company would not have further price hikes this year after it raised prices for three costly cancer therapies a few weeks ago. Another major manufacturer, Merck, announced a 60 percent drop in the price of its hepatitis C treatment Zepatier and will make 10 percent pricing cuts to some other products. News sources report, however, HHS Secretary Azar is not counting on these voluntary actions, “We're driving swift, firm regulatory action and legislative action that's going to create every incentive to bring prices down in this country." A deeper investigation by reporters also found these price decreases were mostly illusory, however, they do show drug companies are working with the administration and likely have no reason to fear future HHS proposals. This brings us to HHS’ first significant proposed rule, currently under review at Office of Management and Budget (OMB).
The End of Rebates As We Know It?
The Office of Inspector General sent a proposed rule to OMB to change the current safe harbor protection for manufacturer rebates paid to insurers and PBMs and establish a new safe harbor rule. Naturally, the proposed rule indicates it would have a substantial impact on the industry of over $100 million. While we don’t have details on the proposed rule at this time, Azar previously testified to Congress he was considering prohibiting rebates to PBMs because, in his view, PBMs whose role is to negotiate discounts can actually benefit from higher list prices. The Pharmaceutical Care Management Association which represents PBMs noted two studies, one from the OIG that found reducing or eliminating the safe harbor for rebates and other discounts would not reduce drug prices. The Pharmaceutical Care Management Association (PCMA) also challenged HHS’ authority to change the safe harbor requirements without congressional action. Depending on the severity of the new rule, a change in drug rebates could have immense implications for PBMs and insurers. It is also unclear which programs the new rule would impact. As Thomas Johnson, Gorman Health Group’s Medicaid expert, noted, “For Medicaid, the drug rebate has been crucial in encouraging states to use Medicaid managed care.”
Drug Importation?
In a third effort to reduce drug costs, the Food and Drug Administration (FDA) announced it will create a working group to explore drug imports to curb drug prices. The FDA will initially focus on imports of drugs when there is a sharp price increase in the U.S. for an off-patent drug produced by a single manufacturer. The Washington Post noted responses from stakeholders that such a limited response would not address the overall trend of increasing drug prices for brand name drugs.
The blueprint talked about increasing competitive pressures for drug prices to come down and the actions this week between the administration and the pharmacy manufacturers and PBMs suggest jaw-boning at least before the election may have an impact even if only temporary and symbolic. However, it should be noted proposed regulations like changing the safe harbor for rebates take months to get to a final rule and the voluntary price reductions are also only temporary – until the end of the year and after the election. The FDA working group will also take time to agree on a strategy to curb imports of drugs that have not been previously approved by the FDA.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Prep for the 2019 Medicare Communications and Marketing Guidelines
I am shocked the 2019 Medicare Marketing Guidelines (MMG) did not come out last Friday since that is when I started vacation. But no… a week later, we are still waiting, and now the wait really starts to impact the development of our marketing strategies and tactics.
In reviewing the April 12, 2018, Centers for Medicare & Medicaid Services (CMS) memo asking for request for input on the 2019 Medicare Communications and Marketing Guidelines, our Marketing team at GHG got together to think about what we would do right now not knowing what the changes are and not being 100% sure if these changes are going into effect. Here are a few suggestions to get your marketing team ready for what we are expecting:
#1 Disclaimers: There is a good chance many of the current disclaimers will be modified and/or deleted. This should not stop you from developing creative materials, although you should have some alternative copy ready to be utilized if the majority of disclaimers go away. Some ideas to consider:
- Laundry list of most important benefits
- Additional “call to action” copy to get prospects to either call you or go to your website
- Copy that clearly states your differential in the market – and it doesn’t matter if this is a repeat of copy. Repeating the most important points you want a prospect to remember is an important strategy!
#2 Font Size: If CMS no longer mandates that all marketing materials have at least a Times New Roman 12-point font type, it will be much easier for the Marketing teams to fit in additional copy points. You may want to develop materials in 11-point and 12-point font to see what extra space you may gain. You may be able to utilize some of the ideas above to help increase marketing points, especially on postcards. Although remember – those whose eyes are over age 65 are not able to read very small type, and if you reverse out the type, it may become illegible, so we would not recommend anything smaller than 11 point font.
#3 Referrals: If CMS allows you to announce that you can offer a gift for a referral and you can request email addresses when asking for referrals in addition to the mailing address – start planning for a mailing now! What free gift could you provide for a referral? Think about allowing members to send referrals to the plan by email, phone, as well as mail so you can utilize these leads quickly for the Annual Election Period (AEP).
#4 Business Reply Cards (BRCs): BRCs that do not mention plan-specific benefits do not need to be submitted into the Health Plan Management System (HPMS). Make sure now that your BRCs do not mention benefits. This is an easy fix—make it now.
#5 Provider Communications: CMS is expected to clarify it is not a violation of CMS marketing requirements if contracted providers notify their patients that the contract status between the provider and the plan/Part D sponsor is changing. We have seen many plans this year developing benefits and products with their providers. Utilizing providers to communicate this relationship during AEP is important. You don’t have to wait for the guidelines to be released on this one – it is already allowed!
Resources:
Benefits are Submitted. What’s Top of Mind for 2019 Marketing and Sales? Learn more
Whether you need help establishing an effective member experience or member communication strategy, cataloging and evaluating existing member communications, or identifying opportunities to streamline and strengthen your member engagement tactics or interventions, we can help. Read more here
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
House Passes Opioid Bill
Surprise, the House actually passed a bipartisan healthcare bill on June 22 that had support from almost all members of both parties. H.R. 6, Support for Patients and Communities Act, is a comprehensive opioid bill that combines more than 58 individual bills intended to address the national opioid epidemic. The bipartisan bill passed 396-14 with only one Democrat voting against it. The impetus was political pressure in an election year to address a problem that results in the death of 115 Americans each day. A recent CBS poll showed 71 percent of all Americans and 78 percent of Republicans support a government response to the crisis.
The bill also passed because it avoided controversy by not including large amounts of new funding. Members from both parties want to show progress on an issue that affects virtually every state before the November elections. Earlier this year, Congress approved $4 billion in new funding, which advocates argue is a drop in the bucket given the scope of the crisis. The House bill does not include any significant new funding, which is a disappointment to Democrats and advocates, however, they supported the bill to show some accomplishment in an election year on an issue important to voters. They are also hopeful there will be other opportunities for additional funding, e.g., in the Senate bill or through the appropriations process. Just before passage, the House bill included a provision to delay Medicare eligibility for end-stage renal disease (ESRD) patients for three months, saving the government $290 million over a decade. The Medicare savings will result in shifting costs to insurers and health plans. The impact on Medicare Advantage (MA) plans should be small since new ESRD beneficiaries are not eligible to enroll in an MA plan except for a small number of members who are already enrolled and age into Medicare.
So what does the bill do? It expands access to treatment, e.g., by allowing nurse practitioners and physician assistants to administer drugs that will avert death from an overdose, it encourages the development of non-opioid treatments following surgery and for pain relief, and it includes steps to stem the flow of illicit drugs through the mail from other countries. There are a number of Medicare and Medicaid provisions on substance use disorders (SUD) in HR 6.
Medicaid
- Conduct a demonstration project to increase provider capacity for substance use treatment and recovery services
- Mandate a beneficiary assignment program that identifies at-risk beneficiaries and assigns them to a pharmaceutical home program
- Require state Medicaid programs to have safety edits in place for opioid refills, monitor concurrent drug prescribing, and monitor antipsychotic prescribing for children
- Continue Medicaid coverage for incarcerated juveniles
- Continue Medicaid coverage for youth in foster care until the age of 26 if they move out of state
- Require the Centers for Medicare & Medicaid Services (CMS) to issue guidance on Neonatal Abstinence Syndrome (NAS) treatment options and require a Government Accountability Office (GAO) study on coverage gaps for pregnant women with SUD
- Provide additional incentives for Medicaid health homes for patients with SUD
Medicare
- Require Part D plans to establish drug management programs for at-risk beneficiaries
- Require e-prescribing for coverage of prescription drugs that are controlled substances under the Medicare Part D program
- Create a pass-through payment extension to encourage the development of clinically superior non-opioid drugs
- Add a review of current opioid prescriptions and, as appropriate, a screening for opioid use disorder (OUD) as part of the Welcome to Medicare initial examination
- Incentivize post-surgical injections as a pain treatment alternative to opioids by reversing a reimbursement cut for these treatments in the Ambulatory Service Center setting
- Provide access to Medication-Assisted Treatment (MAT)
- Evaluate the utilization of telehealth services in treating SUD
On June 20, the House passed a controversial separate bill that would allow Medicaid payment for 30 days during a year for a five-year period for substance abuse treatment at inpatient hospitals with more than 16 beds. Medicaid payment for inpatient substance abuse treatment is currently not allowed under the Institution for Mental Disease (IMD) exclusion policy. The cost of this expansion is estimated at $1 billion.
Next, both of these House-passed opioid bills will go to the Senate where three committees have been developing their own legislative package to deal with the opioid crisis. Many of the Senate provisions are similar to the provisions in the House bill. Expect to see congressional passage of a major opioid legislative package by the fall.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe