Readiness Checklist Outlines Key Operational Requirements
The Centers for Medicare & Medicaid Services (CMS) published its annual Readiness Checklist via HPMS memo on 10/2/2018. As in prior years, the checklist provides a high-level overview of key operational requirements for the coming plan year. Plan Sponsors must communicate any at-risk requirements to their CMS Account Managers. Here we summarize important things to consider as the 2019 plan year approaches:
- CMS is phasing out the Social Security and Health Insurance Claim Numbers and moving to a Medicare Beneficiary Identifier (MBI) by April 2019. Plans must ensure all systems are ready for the transition, including any “home-grown” data repositories (e.g., appeal and grievance databases).
- CMS will be providing Medicare Advantage (MA) and Part D Sponsors access to a precluded providers list after eliminating the provider/prescriber enrollment requirement. Claims from those identified in the precluded provider list must be denied.
- The reinstituted Open Enrollment Period (OEP) not only changes enrollment time frames, it also expands customer service extended hours for 7 days a week, 8:00 am to 8:00 pm, through March 31, starting in 2019.
- Update systems, processes, and training to the new guidelines for Special Election Period (SEP) changes for dual-eligible (DE) and other low-income subsidy (LIS) eligible individuals. Beginning 1/1/2019, DE and LIS individuals will only be able to change plans one time per quarter for the first three quarters with no SEP in the fourth quarter. Many systems are automated to allow these elections to process when received, as through 2018, they are unlimited.
- Health plans should be sure to include customer service, enrollment, and appeals and grievances in their drug utilization controls for opioid management. Staff will need to have scripts and processes in place when members are placed in drug management programs that may impact their access to medication and impact potential disenrollment restrictions.
Ensure your employees are familiar with new guidance from CMS, including the Call Letter; Final Rule and Medicare Communications and Marketing Guidelines. The Readiness Checklist does not convey all guidance changes, and understanding the new rules is critical for Plan Sponsor readiness and compliance.

Gorman Health Group conducts readiness assessments for its clients to help identify any areas of risk related to upcoming plan year preparedness. This is especially important for plans new to the market in 2019. Contact us today for additional information.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
The CMS Fall Conference Highlights
The Centers for Medicare & Medicaid Services (CMS) annual fall conference never misses in providing important information for Sponsors. This year’s conference was held in Baltimore on September 6, 2018. Conference materials can be found on the Compliance Training, Education & Outreach website here. CMS covered many areas, but here I’m focusing on two key areas of the conference: Medicare Communications and Marketing Guidelines (MCMG) updates and appeals and grievances classification best practices.
MCMG
One of the most important clarifying statements from CMS was definitively communicating the MCMG replaces the Medicare Marketing Guidelines (MMG) and any guidance within it. What does that mean? If previous MMG guidance is not dictated in the MCMG, Sponsors should consider that guidance obsolete. While many had assumed that was the case, it’s always better to get clarity straight from the source. This single statement should resolve many looming questions by Sponsors.
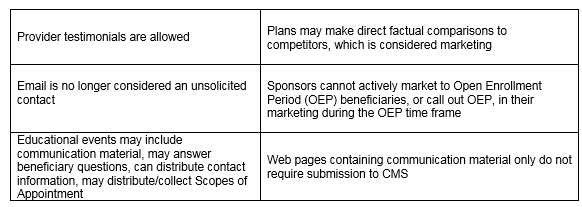
Of equal significance was CMS further outlining the meaning of “marketing” material. In order to be considered “marketing,” the material must meet both intent and content requirements. If only one of these distinctions is met, the material would not be considered “marketing.” The example used to help drive this message was the Evidence of Coverage (EOC). The EOC has content that would meet “marketing” definitions, however, the intent is not to persuade a beneficiary to enroll, so it is considered a communication. This new guidance will eliminate much burden for Sponsors in relation to the number of materials requiring CMS review and submission. While this removes some administrative burden, the oversight may need to be more stringent as health plans are now even more responsible for correct classification of documents and ensuring documents still comply with all CMS requirements. Additionally, documents must be available upon request.
Other noteworthy clarifications from CMS:
An updated MCMG was distributed by CMS on September 6, 2018.
APPEALS & GRIEVANCES
In their continued effort to help Sponsors in the appeal and grievance space, CMS and Sponsor representatives presented classification best practices. CMS reminded Sponsors of key words when classifying appeals and grievances:
Inquiry ----- a question
Grievances ----- a dissatisfaction
Coverage/Organization Determination ----- a decision to provide or pay
Appeal ----- a dispute of a previous decision
Several health plan speakers shared their best practices. Some of the valuable recommendations that changed their organization included:
- Centralized Customer Service staff focused on identifying appeals and grievances. Staff training focused on real-life examples and involved frequent refreshers.
- Cross-functional all staff on grievances, coverage and organization determinations, and appeals. Training not only included correct identification and classification but also the downstream member and CMS impact of failure to correctly identify complaints. This holistic approach supports the member experience and greater CMS compliance.
- Intake staff who review cases quickly to identify any potential misclassification of cases. Root cause analysis as a routine part of processing. So often the focus is on resolving the identified issue for that member, which is critical. Equal importance is remedying any systemic concerns to prevent the problem from impacting other members. The presenters discussed how root cause was discussed in routine meetings with other departments for brainstorming and resolution.
- Embedded Compliance staff within the business units. A great trend in the industry in recent years is to have an operational Compliance person located in critical operations units. They are available to help staff work through complex issues where a keen eye on regulatory guidance is needed. This keeps cases moving forward and allows staff to feel supported all while maintaining high compliance.
CMS conferences have always been valuable resources in clarifying new changes or functions that have the attention of CMS. Don’t miss utilizing this valuable resource to stay current.
 Gorman Health Group provides training tools, conducts mock audits, and supports process improvement initiatives related to Parts C & D appeals and grievances? We also provide interim staff for clients when they have a need in this area.
Gorman Health Group provides training tools, conducts mock audits, and supports process improvement initiatives related to Parts C & D appeals and grievances? We also provide interim staff for clients when they have a need in this area.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Three Costs of Marketing Material Errors
Now that bids have been submitted and the Centers for Medicare & Medicaid Services (CMS) is in the process of finalizing their posted models (check the dates on those versions, people!), it’s time to think about material development and review.
If you are in Compliance or Product Development, chances are you have some responsibility for the material creation and review process. If you have had your hand in the process for a few years, your process has surely evolved, but some things stay the same such as:
- CMS continues to issue enforcement actions for erroneous or late materials.
- Your competitors are always on the lookout for non-compliant and misleading material, and they have no hesitation to contact your Regional Office.
- You nail down a project plan, chock full of deadlines for your creative vendor to provide you versions, for business areas to confirm accuracy of language, to deliver pieces in a staggered manner, and it never seems to stay on track.
CMS is pretty prescriptive when it comes to their model materials. The agency is going through their internal overhaul of the guidelines for Medicare Advantage, Part D, and 1876 Cost plans (released July 20 of last year), and Gorman Health Group is preparing our team to review these ever-important documents for compliance and accuracy. Each year, we support organizations to ensure materials follow CMS’ strict requirements. And with proposed changes to marketing rules (for example, disclaimers), it is more important than ever to have experienced reviewers aiding you in the process.
The costs associated with errors in this area are threefold. The first and most visible are the Civil Money Penalties (CMPs) levied on a handful of sponsors each year. If you are new to the industry (or short-sighted), you might see these CMP notices and think the cost is a drop in the bucket. That’s where I bring you to a second and less visible cost, known only to affected plan sponsors. When errors are identified, there are added internal operations costs, not only in reprint and redistribution fees, but also in staff time to correct erroneous materials in a swift, drop-everything manner.
The third cost, which I believe is the least visible but most important, is the effect this impact takes on enrollees, especially on their perception of your plan. If a member enrolled due to misinformation, this is highly disruptive. This type of issue plants serious misgivings into enrollee and caretaker minds. Materials must be clear and accurate, and with pre-enrollment packet development in mind, you only have one chance to make a first impression.
Trust me – I do not love my own plan deductibles and cost sharing, but the materials provided to me clearly outline my obligations. When I or my family members have been provided inaccurate or incomplete information, I have dropped plans at the first open enrollment period, and I have been vocal about my experience. If you are striving for retention, do not cut corners when it comes to the review of your materials.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
The 2019 Model Marketing Materials Are Posted. Ready for What's Next?
As we await the Medicare Communications and Marketing Guidelines (MCMG), The Centers for Medicare & Medicaid Services (CMS) released the 2019 Model Marketing Materials which includes standardized outreach and educational materials for Medicare Advantage Plans, Medicare Advantage Prescription Drug Plans, Prescription Drug Plans, and 1876 Cost Plans.
Bids are due in a little over a week. You probably have an audit or two going on, and also your day-to-day work needs to be accomplished. CMS will continue to issue enforcement actions for reasons such as delayed and inaccurate materials. Don’t let other activities impact your attention to this area.
Here are three pieces of advice while we wait for the chapter:
- Do not reduce your internal quality control and compliance reviews of materials. While certain pieces will no longer be considered “marketing” and therefore will not require submission to the agency, you should anticipate that CMS (and your competitors) will be on the lookout for misleading and confusing communications.
- Formulate your questions after the release of the MCMG. If something is unclear, ask before you do. I am a huge fan of the adage, “it’s better to say sorry later than to ask permission now,” but I generally save that for when I am encouraging my precocious nephews. I do not recommend employing that strategy when working with Government Programs. The agency often releases FAQs, so get your questions in.
- Continue to plan for your review season. The deadline for plans to provide the ANOC/EOC, formularies, LIS rider and directories is September 30. That means it needs to be in their hands by that time so they can make informed decisions.
Once you have your Plan Benefit Packages in place (we are actively helping with that, too), Gorman Health Group's experienced staff can review your sales collateral to ensure your content is compliant. Our marketing material review service includes:
- Staff members with over 10 years of experience in CMS marketing material review
- A compliance review to ensure model instructions and MCMG are followed
- A benefit review to ensure accuracy of approved Part C and Part D services
- A structured process with quality checkpoints and full project management support
Contact me directly at rpennypacker@ghgadvisors.com for more information.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
In a recent HPMS memo, CMS confirmed the Medicare Marketing Guidelines will be renamed the “Medicare Communication and Marketing Guidelines.” Read more here.
CMS Audit Annual Report Highlights Importance of Mock Audits
Imagine you are the Compliance Officer of your health plan, and you see the email from the Centers for Medicare & Medicaid Services (CMS) announcing you have been selected for a program audit. Did you take the opportunity in the months leading up to this day to confirm your organization can pull compliant universes? Did you take the time to perform some mock reviews with your Organization Determinations, Appeals, and Grievances (ODAG), Coverage Determinations, Appeals, and Grievances (CDAG), Formulary Administration (FA), Special Needs Plan Model of Care (SNP-MOC), if applicable, and Compliance Program Effectiveness (CPE) staff to ensure they are comfortable and skilled presenters? What about your first-tier, downstream, and related entities (FDRs)? Or did you wait, thinking you had plenty of time to get this done?
In the May 8, 2018, release of the Part C and D Program Audit Annual Report (found here), CMS stated, “Mock audits will not only help you prepare for an actual CMS audit, but may help you improve your operations by identifying areas that are problematic or otherwise non-compliant with CMS regulations.”
Our clients find the Gorman Health Group mock audit services provide value by:
- Identifying findings not known at the time of the mock audit
- Providing recommendations on the selection of the best speakers and presenters
- Uncovering gaps in processes, including one plan under the impression the delegate was performing a function that the delegate thought the plan was performing
- Identifying additional FDRs
- Determining a plan was not sending the Explanation of Benefits on a monthly basis
At Gorman Health Group, we can provide your organization with a structured mock program audit experience utilizing our industry veterans and the latest CMS audit protocols, offering a clear picture of how you might perform in a CMS program audit. The mock audit approach also allows you to begin correcting any deficiencies noted before CMS arrives. Are you ready?
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
2018 Audit & Enforcement Conference: Program Audit Changes on the Horizon
On Monday, I provided some highlights of last week’s conferences during our weekly Insider call. The Centers for Medicare & Medicaid Services (CMS) hosted the Spring Conference on May 9 and the Audit & Enforcement Conference on May 10. The Program Audit tools and methodology continue to evolve. The agency gave the industry a heads up about the changes when the data collection tools were posted for public comment. Today I focus on one session, New Approach to 2019 Audits and Universes.
Before the 2019 proposed changes, protocols were made up of audit process and data request documents and questionnaires. CMS also maintained internal information such as how samples are targeted and how Compliance Program elements are assessed. I have outlined some highlighted points from the session:
- CMS refers to the data collection tools as the “what” and refers to the audit process documents as the “how.” Going forward, the industry should see the data request documents released for public comment and the reformatted audit process documents posted on CMS’ Compliance and Audits
- Gone are the Excel impact analysis documents. Instead, CMS will follow the universe record layout for requesting impact data in an effort to make audits more efficient.
- Root cause analysis is still of paramount importance to the agency. Often we see why a specific case fails during a webinar, but determining the overall issue beyond the case-level detail of failure will still be requested.
- Timeliness metrics will be broken out; for example, ODAG timeliness will be evaluated, and sub-element results will be provided such as timeliness of notification. This change could help sponsors better identify root cause and correction steps.
- In addition to the arguably broadest change of consolidating certain CDAG and ODAG universes (still separated by Part C and Part D), the agency removed data points that were no longer meaningful and removed questionnaires that were no longer necessary or were not being used as intended.
- Universe integrity will be extended to all program audit areas. In 2018, the agency will pilot this in a few audits. We expect this to add additional webinar time for the agency, plan sponsors, and delegates. For data integrity webinars, the agency will still select five cases of standard and five cases of expedited (for a total of 10), even though the two case types will be in one table.
Normally, the video recordings would have been posted by this point. However, the agency notes it has a new process for releasing and posting the videos. Once management approval is obtained, registered participants will be notified when the videos are available for viewing. If you were not registered for the conference, periodically check the “Event Archive” webpage for updates. Stay tuned to this page for more insight on these conferences and other agency activity.
Resources:
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Lack of Staff: Biggest Hurdle to Success
Staffing continues to be a major hurdle in the healthcare industry. A recent poll conducted by Gorman Health Group showed that 38% of respondents believed the biggest hurdle to success in their organization was lack of knowledgeable staff or lack of staff.

What is the meaning of success? Is it reaching financial targets? Meeting and exceeding service level agreements? Achieving high marks in customer satisfaction? Being one of the best places to work? Having a great reputation in the community? I am not sure we have seen an organization that is meeting all of these factors. Then again, those are not the organizations that typically call John Gorman for help.
Most places have an ironed-out Human Resources process that includes job description drafting, salary grading, recruitment, and interview process, which may take weeks or sometimes months. However, regulatory agencies wait for no man in terms of expecting compliance metrics to be met. As I observed my colleague tell a group of client trainees, “Your contract is with the federal government.” I can think of no finer way to articulate the commitment made to offer Medicare Advantage and Part D.
Our industry experts currently hold interim staffing positions in all areas, including risk adjustment, compliance, strategy, operations, network management, and pharmacy. While organizations search for their full-time candidate, Gorman Health Group provides experts who have done the job, can manage the department, can report to their C-suite, and much more. Do not let a temporary lack of staff hinder your success. If lack of staff is preventing you from meeting requirements, we can tell you from experience that it by no means sways the Oversight and Enforcement Group from their obligations.
Resources:
Registration is open for the Gorman Health Group 2018 Forum, April 25-26, 2018, at the Red Rock Resort ideally located near the Red Rock Canyon in Las Vegas. Download our agenda here.
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Latest Audit Enforcement Actions Issued by CMS
Like clockwork, the Centers for Medicare & Medicaid Services published the enforcement action notices issued to sponsors related to 2017 program audits. Additional detail regarding conditions, audit scores, and enforcement is expected to be included in the 2017 Program Audit Enforcement Report, which the agency hopes to release before their conferences taking place May 9-10. In the meantime, we break down the published data, which includes not only program audit actions but others as well:
- Eighteen sponsors were issued almost $2.6 million in Civil Money Penalties (CMP) between September 2017 and February 2018 based on their 2017 program audit findings.
- 72% of sponsors cited for Coverage Determinations, Appeals, and Grievances violations
- 61% of sponsors cited for Formulary and Benefit Administration violations
- 39% of sponsors cited for Organization Determinations, Appeals, and Grievances violations
- 22% of sponsors cited for Part C Beneficiary Protections/cost sharing violations
- A Program of All-inclusive Care for the Elderly (PACE) sponsor was issued a CMP in November of 2017, and two PACE sponsors had enrollment suspended in the fourth quarter of 2017.
- PACE plans: You are small but have a mighty sense of responsibility. If you have not done so already, review the posted enforcement notices, distribute within your organization, and create an action plan if you identify any similar findings.
- One Prescription Drug Plan sponsor had enrollment suspended due to medical loss ratio.
- Two sponsors were issued CMPs in 2017 based on outlier status of auto-forwards to the Independent Review Entity.
CMS noted in their draft call letter the agency is considering adding a CMP icon in Medicare Plan Finder (MPF) starting in 2019. If the agency proceeds that way, sponsors undergoing audits this year and incurring CMPs will be impacted by this new indicator. We support efforts such as this which promote beneficiary transparency. As I outlined in our analysis, sponsors should take note. Low Performing Icon information has not been limited to the MPF. Marketing organizations and other industry publications have taken that information and run with it, which may give an advantage to competitors of affected plans. In a recent Bloomberg Law article, I further discuss enforcement actions and the implications of this Low Performing Icon.
Remember that enforcement actions can be levied not just for program audit performance but also for a host of other violations. While I have provided some recent statistics, an analysis of actions taken year over year show patterns in some regards, and no rhyme or reason in other regards. Don’t spend too much time slicing and dicing these figures for your management; let us do that here in these articles. Focus on plan performance and continuous improvement. The goal should be to ensure your organization does not end up with enforcement actions in the first place.
Resources:
Gorman Health Group’s summary and analysis of the 2019 Advance Notice and Draft Call Letter for Medicare Advantage and Part D is now available. Download now
Registration is open for the Gorman Health Group 2018 Forum, April 25-26, 2018, at the Red Rock Resort ideally located near the Red Rock Canyon in Las Vegas. Download our agenda here.
Want to stay up to date on policy and regulation changes? The Insider is GHG’s exclusive intelligence briefing, providing in-depth analysis and expert summaries of the most critical legislative and political activities impacting and shaping your organization. Read our full press release >>
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
Audit Engagement Letters Will Start in March
If you did not have the pleasure of being part of a Centers for Medicare & Medicaid Services (CMS) Program Audit in 2017, don’t be caught off guard if you receive your invitation this year.
Audit engagement letters will start going out this month.
CMS has made few changes to the 2018 CMS program audit protocol from 2017. However, one change was for the Call Log submission for Coverage Determinations, Appeals, and Grievances (CDAG) and Organization Determinations, Appeals, and Grievances (ODAG). With the exception of Medicare-Medicaid Plans (MMPs), the number of call days required to be submitted varies based on the plans sponsors’ enrollment.
While helping plans survive the CMS program audits last year, Gorman Health Group observed one standout area of struggle: call logs. The addition of call logs to the audit protocol relates back to ensuring plan sponsors are appropriately classifying and handling grievances, coverage determinations (Medicare Part C and Medicare Part D), and member notifications. It really boils down to customer service and proving your representatives are handling the cases appropriately. The importance of customer service cannot be stressed enough. At the heart of every business is good customer service. Within the Medicare space, any opportunity to make the member experience a positive one is important from both a quality of care and Star Ratings perspective. Call logs are a means to assess current service levels and to identify training and improvement opportunities. There are now vendors who utilize artificial intelligence to detect the emotions of the caller and how to handle the call appropriately—if the caller is frustrated, they may need to be handled as a grievance.
If you have not established an oversight program or performed a universe pull for call logs, don’t wait any longer! Identifying any issues with data integrity and the service/information provided by your customer service representatives is crucial. Pay particular attention to how the calls are being documented and the reliance on vendor or inter-departmental communications. You want to ensure call transcripts are entered into your system and notes would easily walk an auditor through the case from the time the call was answered through to resolution and that it has been sufficiently documented. If there is a gap in your current process, it is time to put a plan in place.
Gorman Health Group can assist your plan with mock audit services ranging from a complete program audit to a specific, targeted audit of your call logs. The time to act is now to avoid getting caught with your pants down.
Resources:
Gorman Health Group’s summary and analysis of the 2019 Advance Notice and Draft Call Letter for Medicare Advantage and Part D is now available. Download now
Registration is open for the Gorman Health Group 2018 Forum, April 25-26, 2018, at the Red Rock Resort ideally located near the Red Rock Canyon in Las Vegas. Download our agenda here.
Want to stay up to date on policy and regulation changes? The Insider is GHG’s exclusive intelligence briefing, providing in-depth analysis and expert summaries of the most critical legislative and political activities impacting and shaping your organization. Read our full press release >>
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe
CMS Focus: Compliant Independent Review Entity Data
The focus on compliant Independent Review Entity (IRE) data should come as no surprise to Part D sponsors. In December 2016, the Centers for Medicare & Medicaid Services (CMS) released the Health Plan Management System (HPMS) memo, Compliance and Enforcement Actions Related to Part D Auto-Forwards, indicating sponsors with inordinately high auto-forward rates were subject to compliance actions that could be escalated to enforcement actions. The memo established a threshold rate, and in spring of 2017, CMS began imposing civil monetary penalties (CMPs) on Part D sponsors with demonstrated non-compliance with coverage determination and redetermination auto-forwards to the IRE. And with that, the die was cast.
The Timeliness Monitoring Project (TMP) is an opportunity to demonstrate compliant processes and data integrity in support of CMS’ Star Ratings. With this data collection effort, the playing field is leveled in the evaluation of sponsors’ IRE data. The TMP effort by CMS – while offering a fair and balanced methodology as it seeks to assess all plans instead of those identified in a targeted review – also provides CMS a separate and distinct window beyond program audits in identifying sponsors with problematic organization and coverage determination processes.
Plans that are unable to provide complete and accurate universes will be at risk with both their Part C and Part D Star Ratings as described in the December 12, 2017, HPMS memo, Timeliness Monitoring Project (TMP). “CMS considers data integrity issues, if identified, as an indicator that a contract’s measure data are invalid for the Star Ratings. CMS may also independently evaluate the data to gain insight into sponsors’ performance in these two program areas.”
The unfortunate reality is that an inability to accurately capture data within organization systems is likely symptomatic of any number of inefficiencies, for example:
- Lack of systems/analytics to compile data needed to assess compliance with CMS expectations
- Insufficient monitoring efforts
- Potential for processing inefficiencies such as inadequate resources, training, or expertise
CMS proposes a scaled reduction in a sponsor’s Star Ratings data that is found to be incomplete or “lack integrity.” The consequences of a lowered Star Rating can be a devastating blow to sponsors. Yet often, performance issues remain inadequately addressed. Plans sometimes need help knowing where to begin.
Sponsors can readily utilize the Audit Process and Data Request guidance for Organization Determinations, Appeals, and Grievances (ODAG) and Coverage Determinations, Appeals, and Grievances (CDAG) as the playbook on IRE auto-forward compliance.
Start with a great outreach process
- Is plan staff aware of what clinical information is required to make a well-informed decision?
- Is there a consistent, timely, and well-documented process in place for provider outreach?
- Can this be evidenced in your systems, and more importantly, is this being tracked and monitored by plan staff?
Follow up with timely and sound decision-making
- Do the decision-makers have all the information they need to make the decision?
- Are decisions primarily made based on sponsor formulary/Evidence of Coverage (EOC), clinical criteria, federal regulations, CMS guidance, compendia, or peer-reviewed literature (where allowed)?
- Are there any trends in plan denials for lack of clinical information? What efforts are being made to address those trends?
Ensure adequate notification processes exist
- Plans should have well-established processes for enrollee and provider notification that includes consistent methods of outreach, clear and unambiguous documentation in systems, with well-written and understandable denial rationale.
- Notifications must be timely, with documentation of both oral and written outreach detailed in plan systems. Plans must be able to evidence when the notification(s) entered the mail stream.
Sponsors have the ability to ready themselves by:
- Regular monitoring through sample mock auditing
- Developing dashboard reporting to assess the veracity of the data
- Evaluating universe creation and testing it: can all steps be evidenced in plan systems?
- Ensure internal processes for organization determinations and reconsiderations/coverage determinations and redeterminations are working effectively
Gorman Health Group’s Clinical Solutions practice area has a talented team of registered nurse professionals with experience in operations and implementations in various healthcare lines of businesses. Add to that Gorman Health Group’s Compliance consulting expertise and data analysts, and you have the winning combination in driving better member outcomes and ensuring member satisfaction. One call can lead to the answers your plan is seeking!
Resources:
Gorman Health Group’s summary and analysis of the 2019 Advance Notice and Draft Call Letter for Medicare Advantage and Part D is now available. Download now
Registration is open for the Gorman Health Group 2018 Forum, April 25-26, 2018, at the Red Rock Resort ideally located near the Red Rock Canyon in Las Vegas.
Want to stay up to date on policy and regulation changes? The Insider is GHG’s exclusive intelligence briefing, providing in-depth analysis and expert summaries of the most critical legislative and political activities impacting and shaping your organization. Read our full press release >>
Stay connected to industry news and gain perspective on how to navigate the latest issues through GHG’s weekly newsletter. Subscribe